Suitable animal models are essential for translational research, especially in the case of complex, multifactorial conditions, such as obesity. In this study, scientists of the Research Group Epigenetics, Metabolism and Longevity, Dummerstorf and the German Mouse Clinic, Helmholtz Zentrum München, characterized the geno- and phenotypes of the mouse line Titan and tested its suitability as an interventional obesity model. The results of this research were recently published in Communications Biology.
The non-inbred mouse line Titan, also known as DU6, is one of the world’s longest selection experiments for high body mass. The unique model results from an ongoing long-term breeding scheme (over 180 generations) that selected mice based on high body mass at six weeks of age. The unselected population was maintained as a control group throughout the selection process. In contrast to genetically homogeneous inbred lines, that are useful because of the limited inter-animal variability, a model based on the selection of phenotypic traits, in this case obesity, may provide novel insight into complex disorders.
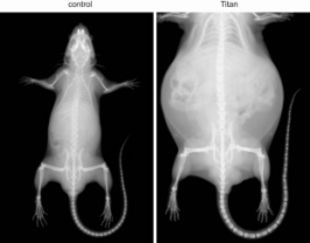
Phenotypic characterization revealed that Titan mice are severely obese, giant and short lived. Titan male mice had an average weight of 90 g at the time of selection (6 weeks of age) vs. 35 g for unselected control mice. Adult mice further grew to an average of over 110 g. Titan mice exhibited an increase in both total fat and lean mass, as well as in fat percentage. Under standard breeding feed (SBF), Titan mice reached 10 and 50% mortality at 125 and 325 days, respectively. In comparison, control mice showed double mean lifespan, reaching 10 and 50% death at 307 and 645 days, respectively. The shortened lifespan of Titan mice combined with obese metrics suggests that the Titan mice suffer from detrimental obesity, rather than benign obesity.
Line-specific patterns of genetic invariability are in accordance with observed phenotypic traits. Regions of distinct genetic differentiation (RDD) unique to Titan mice comprise 84 genes. The data support a link between Titan-specific RDD such as metabolic regulation, growth, skin differentiation, and immune regulatory genes with Titan-specific phenotypes of obesity, size, thicker dermis, and increased hallmarks of inflammation. Titan mice also showed modifications in the liver transcriptome, proteome, and epi-genome linked to metabolic (dys)regulations.
The dietary intervention performed in this study partially reversed the metabolic phenotype in Titan mice and significantly prolonged their lifespan. Energy reduced food (ERF) intervention resulted in a 5–10% weight reduction in Titan mice, compared to SBF-fed controls. By 21 weeks, ERF-fed Titan mice had significantly reduced abdominal fat percentage compared to SBF-fed mice. The ERF regime lowered the levels of plasma cholesterol, HDL, and glucose in Titan mice. Most important, the ERF diet significantly increased the lifespan of Titan mice. ERF-fed Titan mice reached 10 and 50% death at 219 and 374 days, respectively, which is significantly longer than for the SBF-fed Titan mice (10% death at 125 days and 50% death at 325 days).
The detailed genomic, epigenetic, and phenotypic analyses described support the Titan line as a relevant model to study various aspects of interventions in unhealthy obesity. The present phenotypic analysis provides a solid basis for further characterization of the Titan mouse model as a novel tool for studying and potentially developing pharmaceutical interventions targeting obesity and associated disorders.
Dietary intervention improves health metrics and life expectancy of the genetically obese Titan mouse. Müller-Eigner A, Sanz-Moreno A, de-Diego I, Venkatasubramani AV, Langhammer M, Gerlini R, Rathkolb B, Aguilar-Pimentel A, Klein-Rodewald T, Calzada-Wack J, Becker L, Palma-Vera S, Gille B, Forne I, Imhof A, Meng C, Ludwig C, Koch F, Heiker JT, Kuhla A, Caton V, Brenmoehl J, Reyer H, Schoen J, Fuchs H, Gailus-Durner V, Hoeflich A, de Angelis MH, Peleg S. Commun Biol. 2022 May 3;5(1):408. doi: 10.1038/s42003-022-03339-3. PMID: 35505192; PMCID: PMC9065075.